Chemistry Chemistry questions and answers which statement about surface tension is false. 1. Liquids tend to minimize their surface area 2. molecules on the surface of the liquid have fewer molecules to interact with 3. Increase intermilecular forces increase surface tension 4. Items with density lower than water will sink due to surface tension 5.
IJERPH | Free Full-Text | “In Flow”! Why Do Users Share Fake News about Environmentally Friendly Brands on Social Media?
Jun 23, 20223.1: What is surface tension? Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary depending on the nature of the liquid (e.g. water vs. gasoline) or solutes in the liquid (e.g. surfactants like detergent), each solution exhibits differing

Source Image: amazon.com
Download Image
Jan 30, 2023Surface Tension. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary depending on the nature of the liquid (e.g. water vs. gasoline) or solutes in the liquid (e.g. surfactants like detergent), each solution exhibits differing surface

Source Image: amazon.com
Download Image
Modal Analysis – Minimum Initial Strain | Dlubal Software For a stable interface of any two fluids, γ is always positive. 8 For surfaces of typical liquids (or their interfaces with air), at room temperature, the surface tension equals a few 10 − 2 J / m2, corresponding to the potential energy Ui of a few 10 − 2eV per surface molecule – i.e. just a fraction of the full binding (or “cohesive

Source Image: studypool.com
Download Image
Which Statement About Surface Tension Is False
For a stable interface of any two fluids, γ is always positive. 8 For surfaces of typical liquids (or their interfaces with air), at room temperature, the surface tension equals a few 10 − 2 J / m2, corresponding to the potential energy Ui of a few 10 − 2eV per surface molecule – i.e. just a fraction of the full binding (or “cohesive Place the penny, heads up, on top of a paper towel. 3. Hold your dropper about 1-inch above the penny and add drops of water to the surface of the penny until it overflows. 4. Record the number of drops of water the surface of the penny can hold in the table on the next page under the column labeled “Run 1.”. 5.
SOLUTION: Yhlghcomanisfsbofj60 1 – Studypool
1. tendency of the lung to recoil because of its elastic properties and the surface tension of the alveolar fluid. 2. tendency of he compressed chest wall to recoil and expand outward. these two forces pull the lungs away from the thoracic wall, creating a partial vacuum in pleural cavity. Surface Tension: Formula Sheet #hydrostatics #physics | Surface tension, Physics notes, Study notes

Source Image: in.pinterest.com
Download Image
Solved Question 3 (1 point) Which of the following | Chegg.com 1. tendency of the lung to recoil because of its elastic properties and the surface tension of the alveolar fluid. 2. tendency of he compressed chest wall to recoil and expand outward. these two forces pull the lungs away from the thoracic wall, creating a partial vacuum in pleural cavity.

Source Image: chegg.com
Download Image
IJERPH | Free Full-Text | “In Flow”! Why Do Users Share Fake News about Environmentally Friendly Brands on Social Media? Chemistry Chemistry questions and answers which statement about surface tension is false. 1. Liquids tend to minimize their surface area 2. molecules on the surface of the liquid have fewer molecules to interact with 3. Increase intermilecular forces increase surface tension 4. Items with density lower than water will sink due to surface tension 5.
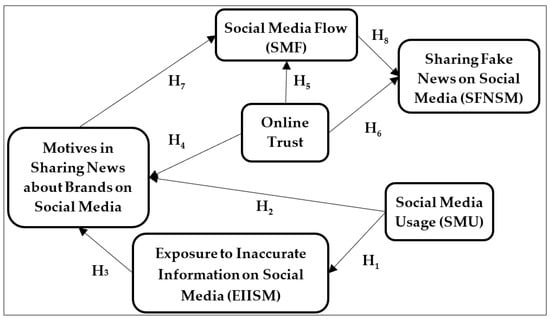
Source Image: mdpi.com
Download Image
Modal Analysis – Minimum Initial Strain | Dlubal Software Jan 30, 2023Surface Tension. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary depending on the nature of the liquid (e.g. water vs. gasoline) or solutes in the liquid (e.g. surfactants like detergent), each solution exhibits differing surface

Source Image: dlubal.com
Download Image
SOLUTION: Yhlghcomanisfsbofj60 1 – Studypool Sep 21, 2022It is also responsible for the beading up of water droplets on a freshly waxed car because there are no attractions between the polar water molecules and the nonpolar wax. Figure 13.6.2 13.6. 2: (A) Molecules at the surface of a liquid are pulled downwards into the liquid, creating a tightened surface. (B) Surface tension allows a paper clip to

Source Image: studypool.com
Download Image
Note Library – Surface Tension #ioeentrancehub | Facebook For a stable interface of any two fluids, γ is always positive. 8 For surfaces of typical liquids (or their interfaces with air), at room temperature, the surface tension equals a few 10 − 2 J / m2, corresponding to the potential energy Ui of a few 10 − 2eV per surface molecule – i.e. just a fraction of the full binding (or “cohesive

Source Image: facebook.com
Download Image
PhysioEx Exercise 7 Activity 3 – PhysioEx Lab Report Exercise 7: Respiratory System Mechanics – Studocu Place the penny, heads up, on top of a paper towel. 3. Hold your dropper about 1-inch above the penny and add drops of water to the surface of the penny until it overflows. 4. Record the number of drops of water the surface of the penny can hold in the table on the next page under the column labeled “Run 1.”. 5.

Source Image: studocu.com
Download Image
Solved Question 3 (1 point) Which of the following | Chegg.com
PhysioEx Exercise 7 Activity 3 – PhysioEx Lab Report Exercise 7: Respiratory System Mechanics – Studocu Jun 23, 20223.1: What is surface tension? Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary depending on the nature of the liquid (e.g. water vs. gasoline) or solutes in the liquid (e.g. surfactants like detergent), each solution exhibits differing
Modal Analysis – Minimum Initial Strain | Dlubal Software Note Library – Surface Tension #ioeentrancehub | Facebook Sep 21, 2022It is also responsible for the beading up of water droplets on a freshly waxed car because there are no attractions between the polar water molecules and the nonpolar wax. Figure 13.6.2 13.6. 2: (A) Molecules at the surface of a liquid are pulled downwards into the liquid, creating a tightened surface. (B) Surface tension allows a paper clip to